| RBGPF | 1.33% | 61 | $ | |
| BCE | -2.04% | 26.48 | $ | |
| VOD | -0.62% | 8.855 | $ | |
| CMSC | -0.65% | 24.57 | $ | |
| RIO | -1.85% | 61.835 | $ | |
| NGG | -0.92% | 62.683 | $ | |
| BCC | -2.98% | 148.08 | $ | |
| SCS | -1.11% | 13.57 | $ | |
| RYCEF | 0.44% | 6.8 | $ | |
| RELX | 0.21% | 46.67 | $ | |
| CMSD | -0.71% | 24.407 | $ | |
| BTI | 0.55% | 37.535 | $ | |
| AZN | -0.42% | 66.12 | $ | |
| GSK | -0.81% | 33.875 | $ | |
| BP | -1.73% | 28.82 | $ | |
| JRI | -0.15% | 13.35 | $ |

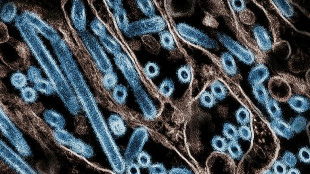
US tells pharmas to make Covid boosters targeting BA.4 and BA.5
The US Food and Drug Administration on Thursday told vaccine makers that Covid boosters for this fall and winter should include components targeting the BA.4 and BA.5 sublineages of Omicron.
Earlier this week, a panel of medical experts convened by the agency voted in favor of updating Covid vaccines against Omicron, with most indicating they would favor shots that target the latest iterations rather than its original form, BA.1, fearing the latter would be too out-of-date.
BA.4 and BA.5, which are more transmissible and immune evasive, now comprise more than 52 percent of US Covid cases, according to an official tracker.
"We have advised manufacturers seeking to update their Covid-19 vaccines that they should develop modified vaccines that add an omicron BA.4/5 spike protein component to the current vaccine composition to create a two component (bivalent) booster vaccine," the FDA said in a statement.
These vaccines would also need to target the original Wuhan strain, in order to increase the breadth of immune response.
Pfizer and Moderna, which produce messenger RNA Covid vaccines, have developed and tested vaccines against BA.1, and representatives of both companies indicated during the experts' meeting they would need around three months to produce BA.4 and BA.5 vaccines at scale.
Pfizer shared early results showing its BA.4/5 vaccine produced a strong antibody response in mice, but it hasn't yet been trialed in humans.
Novavax, which makes a protein subunit vaccine, said it could offer BA.4/5 vaccines by the end of the year.
The FDA said in its new statement that the companies would need to submit human data prior to authorization.
The "primary series" or first shots a person receives would remain against the original strain, the FDA added.
While previous "variants of concern" like Alpha and Delta eventually petered out, Omicron and its sublineages have dominated throughout 2022, to the point it comprises the vast majority of all Covid in the world, FDA official Jerry Weir told the expert meeting this week.
This makes it more likely that the virus's future evolution will also occur along the Omicron branch of the Covid family tree, he added.
Earlier this month, the World Health Organization also recommended the use of Omicron boosters after a primary series against the original strain.
E.Accardi--IM